
Chaperones catalyze protein folding without being a part of the folded protein. Notably, chaperones do not relay any additional information required for the folding of polypeptides the native conformation of a protein is determined solely by its amino acid sequence. When the amino acids cannot form these interactions, the protein cannot fold by itself and needs chaperones. The native conformation of a protein is formed by interactions between the side chains of its constituent amino acids. The substrate protein, whether folded or not, is released from the chamber. The interior of the chamber is lined with hydrophilic surfaces, where the protein can fold in isolation.ĪTP hydrolysis weakens the binding of the cap and binding of additional ATP molecules ejects the cap. Once the protein is inside, ATP binding seals the chamber with a cap.
This initial binding often helps to unfold a misfolded protein. coli, the chaperonin system is called GroEL/GroES, while its eukaryotic analog is called Hsp60.Ī misfolded protein is captured by hydrophobic interactions with the exposed surface of the opening. If folding does not occur rapidly enough, the polypeptide may bind again, and the process is repeated until the protein is folded into its native conformation.Īlternatively, a fully synthesized and partially folded polypeptide may be delivered to a chaperonin.Ĭhaperonins are large barrel-shaped protein complexes that provide an isolated chamber for protein folding, with one half of the symmetric barrel operating on a client protein at a time. Next, ATP binds the complex again inducing the dissociation of hsp70 and releasing the bound polypeptide, allowing it a chance to re-fold. As a result, parts of hsp70 come together like jaws, trapping the unfolded protein inside. The hsp70 machinery often acts before the protein leaves the ribosome, with each ATP-bound monomer recognizing a small stretch of hydrophobic amino acids on a protein’s surface.Ī set of smaller hsp40 proteins interacts with this complex and triggers ATP hydrolysis. Such patches on different protein molecules can bind to each other, leading to irreversible protein aggregation.Ĭhaperones recognize these exposed hydrophobic patches and prevent protein aggregation, facilitating the folding of the proteins. In misfolded proteins, hydrophobic patches are exposed.
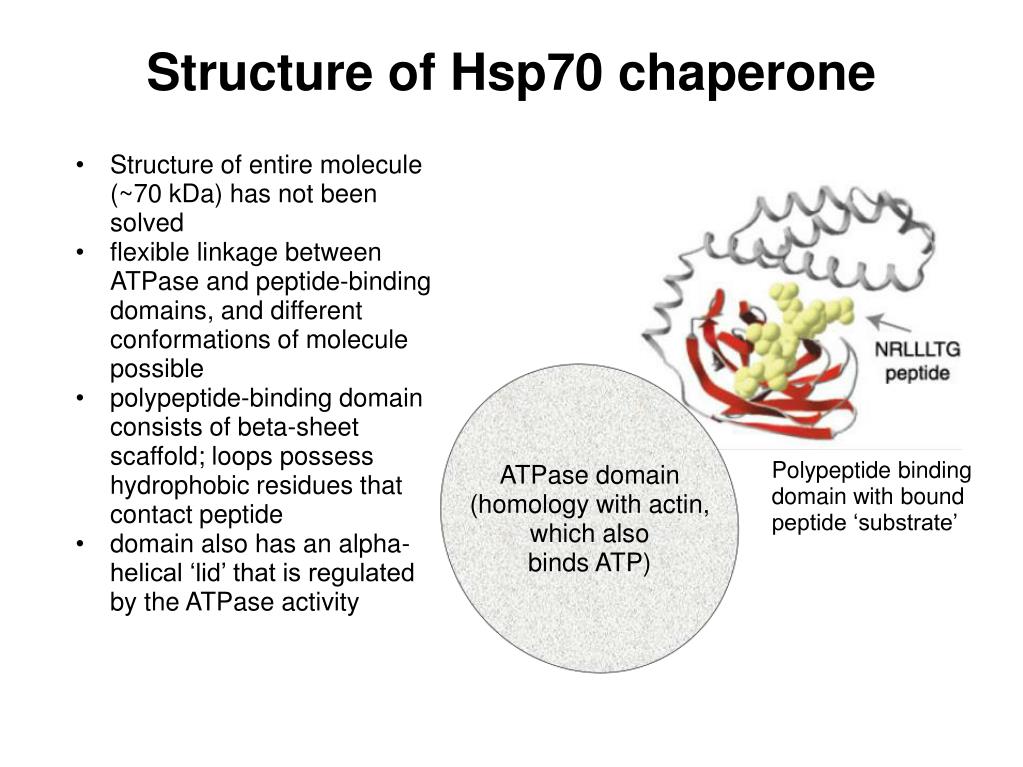

In a correctly folded protein, hydrophobic patches are buried in the interior. Of the several types of molecular chaperones found in prokaryotes and eukaryotes, the two major families are the heat shock proteins - hsp70 and hsp60. As they are synthesized in the cell, most proteins do not fold spontaneously into their native conformation but require a special class of proteins called chaperones to help fold them.


 0 kommentar(er)
0 kommentar(er)
